X-chromosome inactivation is a fascinating biological process that allows female mammals to balance gene dosage by silencing one of their two X chromosomes. This crucial mechanism is responsible for preventing overexpression of X-linked genes, a phenomenon that could lead to various genetic disorders. Notably, recent research spearheaded by Jeannie Lee focuses on understanding the intricacies of X-chromosome inactivation and its implications for diseases such as Fragile X Syndrome and Rett Syndrome. By uncovering the role of the Xist molecule and its interaction with chromosomal environments, Lee’s team is paving the way for breakthroughs in targeted therapies that could significantly improve treatment outcomes for affected individuals. The potential to unsilence inactivated genes not only offers hope for gene therapy but also highlights the importance of ongoing studies in the genetic disorders landscape.
The process of silencing an X chromosome, known as X-inactivation, serves as a vital mechanism in female mammals, ensuring that one copy of the X chromosome remains functional while the other is effectively turned off. This phenomenon is critical in maintaining genetic balance and preventing disorders linked to excessive gene expression. Research led by Jeannie Lee has provided invaluable insights into the factors influencing X-inactivation, particularly the role of the Xist molecule. As new therapeutic avenues emerge, such as those targeting the restoration of gene function for conditions like Fragile X Syndrome and Rett Syndrome, understanding how this genetic regulation occurs becomes crucial. With advancements in medical science, the quest to optimize genetic disorder treatments continues to evolve, bearing the promise of improved health outcomes.
Understanding X-Chromosome Inactivation
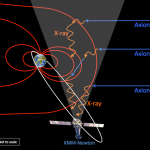
X-chromosome inactivation (XCI) is a critical biological process that occurs in female mammals, leading to the silencing of one of the two X chromosomes present in each cell. This regulatory mechanism ensures that females, having two X chromosomes, do not have double the gene dosage compared to males, who have only one. Jeannie Lee’s groundbreaking research has shed light on the intricate details of this process, investigating how the XIST gene produces RNA molecules that bind to the X chromosome, ultimately altering the properties of the surrounding chromatin—a gelatinous-like substance known as ‘Jell-O.’ This unique interaction is essential for the proper inactivation of the chromosome, facilitating a delicate balance of gene expression within the cell.
Understanding X-inactivation not only contributes to our knowledge of female biology but also holds significance in the context of genetic disorders linked to the X chromosome. The implications of XCI on diseases such as Fragile X Syndrome and Rett Syndrome are profound, since defective genes on the inactivated X can lead to severe developmental issues. By elucidating this cellular mechanism, researchers like Lee are paving the way towards innovative therapies aimed at ‘unsilencing’ these genes, potentially offering new avenues for treatment and improving the quality of life for affected individuals.
The Role of Xist Molecule in Gene Regulation
The Xist molecule plays a pivotal role in the process of X-chromosome inactivation. By producing a long non-coding RNA, Xist spreads along the length of the X chromosome, which triggers the structural changes in the chromatin that lead to inactivation. As Jeannie Lee’s research indicates, Xist interacts with the chromatin’s biophysical properties, modifying it from a rigid state to a more fluid one. This transformation allows other regulatory molecules to access regions of the chromosome that were previously closed off, effectively silencing potentially harmful gene expressions that could lead to disorders such as Fragile X Syndrome and Rett Syndrome. This mechanism not only illustrates the sophistication of gene regulation but also provides insights into targeted therapeutic strategies.
Furthermore, the research into the Xist molecule’s function emphasizes its potential as a therapeutic target for genetic disorders. Many individuals with X-linked conditions are affected by gene mutations on the X chromosome, and releasing the silenced genes could reactivate their healthy counterpart. Lee’s laboratory has initiated significant strides in exploring these therapeutic avenues, aiming to harness the unique properties of the Xist molecule to develop treatment protocols that can safely reactivate silenced genes, addressing the root causes of these genetic disorders. By continuing to optimize these strategies, there is a hopeful trajectory towards clinical applications that could transform treatment for those with Fragile X Syndrome and Rett Syndrome.
Innovations in Treating Fragile X and Rett Syndromes
Recent developments in genetic therapies have opened exciting pathways for treating disorders such as Fragile X Syndrome and Rett Syndrome, both of which are impacted by mutations on the X chromosome. Jeannie Lee’s research team has been at the forefront of exploring how methods to manipulate X-inactivation can lead to breakthroughs in treatment. By understanding the mechanisms behind XCI, researchers are developing techniques that aim to unsilence the genes responsible for these conditions. This innovative approach may allow the healthy version of the gene to be expressed even in individuals where it was previously inactive.
In particular, the potential for XCI-based therapies offers hope not just for females but also for males who are affected by the consequences of X-linked mutations. While males typically only have one X chromosome, the silencing of particular genes can occur due to other mechanisms that mirror X-inactivation. By leveraging knowledge from Jeannie Lee’s research, scientists are exploring ways to reverse these silencing effects, which could increase gene expression in all affected individuals, paving the way for ground-breaking treatment options in the near future.
Genetic Disorders: Impact of Breakthroughs in Research
The advances in understanding X-chromosome inactivation are significant in the context of genetic disorders broadly. The research initiatives led by Jeannie Lee and her team at Harvard Medical School are not just adding to the fundamental biological knowledge but are crucial in shaping future treatments for a range of genetic disorders associated with the X chromosome. This line of inquiry reflects a paradigm shift in how we address genetic conditions, moving from symptom management to potentially correcting the underlying genetic causation.
As a consequence of these discoveries, we might anticipate the emergence of advanced therapies that target the molecular and cellular dysfunctions responsible for disorders like Fragile X and Rett Syndromes. These potential treatments could vastly improve the lives of those affected by ensuring that the healthy genes encapsulated within inactivated X chromosomes can be expressed when needed. The hope is that ongoing research will lead to a better understanding of these complex diseases, ultimately providing tangible therapeutic options and transforming the landscape of genetic disorder treatment.
The Future of Genetic Research: Jeannie Lee’s Vision
Jeannie Lee’s vision for the future of genetic research and therapy is both ambitious and promising. With her extensive background in genetics, Lee has spearheaded initiatives that delve deep into the mechanisms governing X-chromosome inactivation. Her work has not only attempted to answer foundational questions long posed by researchers but has also translated those insights into potential therapeutic strategies that could benefit millions affected by genetic disorders. The transition from basic research to clinical applications is a testament to her commitment to advancing the field.
Moreover, as we witness an unprecedented surge in genetic research breakthroughs, the need for funding and public support for such initiatives has never been more critical. Lee’s hope is to cultivate a greater understanding of genetic conditions by engaging both the scientific community and the public in discussions about the implications of her findings. By fostering collaborations and encouraging interdisciplinary approaches, Lee envisions a future where genetic disorders can be effectively treated, enhancing the quality of life for those affected and shaping a new era of medicine.
Understanding How Treatment Options Evolve
Treatment options for genetic disorders such as Fragile X Syndrome and Rett Syndrome have been limited historically, primarily focused on symptomatic relief rather than targeting the root causes of these conditions. However, the recent breakthroughs in understanding the molecular mechanisms of X-inactivation suggest that the landscape of treatment may soon change dramatically. With ongoing research led by prominent figures like Jeannie Lee, the prospect of therapies that can reactivate silenced genes presents an exciting avenue for driving progress. These innovations can lead to more effective interventions that directly tackle the genetic mutations causative of these disorders.
As the scientific community continues to explore these novel therapeutic strategies, it is crucial for researchers to not only innovate but also to navigate the ethical and safety considerations inherent in gene therapy. Rigorous preclinical studies will pave the way towards safe and effective treatments that can withstand the scrutiny necessary for clinical application. The evolution of treatment protocols will hinge on a collective effort to understand the complexities of genetic disorders at a cellular level to ensure a future where affected individuals have access to the care they desperately need.
The Role of Clinical Trials in Genetic Research
A critical aspect of translating research findings into clinical practice involves the conduct of clinical trials. These studies are essential for assessing the safety and efficacy of new treatments derived from advances in genetic research, such as those pioneered by Jeannie Lee’s lab. As they prepare to move their promising approaches into clinical trials, a wide array of questions will arise regarding treatment protocols, patient selection criteria, and the metrics for measuring success, all of which must be meticulously defined. Participating in clinical trials represents an important opportunity for patients with genetic disorders, as it allows for access to cutting-edge therapies that may not yet be available in standard practice.
Moreover, clinical trials are instrumental in gathering data that can inform future research directions and refine existing knowledge around genetic disorders. The insights gained from these studies can inform subsequent iterations of treatment approaches, contributing to a better understanding of the complexities of gene therapy. It is through this iterative process that effective therapies for Fragile X Syndrome, Rett Syndrome, and other X-linked conditions could transform lives, ensuring that progress in genetic research leads to tangible benefits for patients.
Navigating Ethical Considerations in Genetic Therapy
With advancements in genetic research and the prospect of therapies that modify gene expression, there arises a critical need to address the ethical considerations surrounding these developments. As seen with the potential treatments for Fragile X Syndrome and Rett Syndrome, the implications of manipulating genetic material can be profound. Researchers like Jeannie Lee are not only focused on the science but also on navigating the complex ethical landscape that genetic therapies present. Ensuring the safety of patients and the responsible use of such technologies is paramount as we move closer to clinical application.
Ethical dilemmas may include questions around consent, the long-term impacts of therapies, and the societal implications of gene editing technologies. As genetic treatments advance, it is essential for ongoing dialogues among scientists, ethicists, and the public to consider the wider effects on individuals and communities. By fostering a culture of ethical vigilance and responsibility in the field, researchers can help ensure that the breakthroughs in genetic therapy translate into equitable and beneficial outcomes for all individuals afflicted by genetic disorders.
The Promise of Genetic Research in Transforming Healthcare
The ongoing research and advancements in genetics hold significant promise for transforming healthcare, particularly for individuals with genetic disorders. As exemplified by the work of Jeannie Lee and her focus on X-chromosome inactivation, there is a renewed optimism regarding the development of targeted therapies that could significantly improve the quality of life for those living with conditions like Fragile X Syndrome and Rett Syndrome. This scientific innovation not only enhances our understanding of complex genetics but also empowers future treatment options that could redefine healthcare outcomes.
Furthermore, the implications of these breakthroughs extend beyond individual treatments. As researchers harness the knowledge gained from genetic studies, the potential for broader applications in precision medicine arises. This paradigm shift towards personalized healthcare ensures that treatments are tailored to the unique genetic makeup of individuals. By continuing to invest in and support genetic research initiatives, there lies an immense opportunity to harness the power of genetics in a way that could lead to revolutionary changes in the treatment and understanding of genetic disorders and beyond.
Frequently Asked Questions
What is X-chromosome inactivation and how does it relate to genetic disorders like Fragile X syndrome?
X-chromosome inactivation (XCI) is a biological process where one of the two X chromosomes in females is silenced, preventing the overexpression of X-linked genes. This mechanism is crucial because many genetic disorders, such as Fragile X syndrome, are caused by mutations on the X chromosome. Understanding XCI can lead to breakthroughs in treatment for these disorders.
How does Jeannie Lee’s research contribute to our understanding of X-chromosome inactivation?
Jeannie Lee’s research at Harvard Medical School focuses on the mechanisms of X-chromosome inactivation, detailing how the Xist molecule interacts with chromosomal structures to silence Gene expression. Her findings offer insights that could pave the way for developing treatments for conditions like Fragile X syndrome and Rett syndrome.
What role does the Xist RNA molecule play in X-chromosome inactivation?
The Xist RNA molecule is critical for X-chromosome inactivation. It modifies the gelatinous material surrounding the X chromosome, facilitating the silencing process. By altering this ‘Jell-O-like’ substance, Xist ensures that the X chromosome remains inactive, which is vital for maintaining gene dosage balance in female cells.
Can understanding X-chromosome inactivation lead to breakthroughs in treating Rett syndrome?
Yes, understanding X-chromosome inactivation is essential for treating Rett syndrome. By exploring ways to unsilence the genes on the inactivated X chromosome, researchers like Jeannie Lee aim to restore gene function in patients, which could lead to new therapeutic approaches for Rett syndrome and similar genetic disorders.
What advancements in genetic disorders have resulted from research on X-chromosome inactivation?
Recent advancements in understanding X-chromosome inactivation have catalyzed breakthroughs in treating genetic disorders like Fragile X syndrome and Rett syndrome. Researchers have developed methods to potentially reactivate the healthy genes located on inactivated X chromosomes, offering hope for effective therapies in the near future.
How could treatments based on X-chromosome inactivation benefit males with genetic disorders?
Treatments derived from the mechanisms of X-chromosome inactivation could benefit males, despite their having only one X chromosome. Since mutations can silence specific genes, strategies that target the unsilencing of these genes could restore their function, addressing conditions like Fragile X syndrome in affected males.
What ongoing research is there in the treatment of Fragile X syndrome related to X-chromosome inactivation?
Ongoing research, particularly from Jeannie Lee’s lab, focuses on optimizing methods to unsilence X-linked genes in cells affected by Fragile X syndrome. These approaches include safety studies aimed at paving the way for clinical trials that may lead to actionable therapies for patients with this genetic disorder.
| Key Point | Details |
|---|---|
| X-Chromosome Inactivation | Females have two X chromosomes, while males have one. This necessitates the inactivation of one X chromosome in females to balance gene dosage. |
| Role of Xist Gene | A gene on the X chromosome, Xist, produces an RNA molecule that guides the inactivation process by altering the physical properties of surrounding chromatin. |
| Jell-O-Like Substance | The ‘Jell-O’ substance refers to the chromatin structure that surrounds chromosomes, preventing tangled chromosomes and facilitating the inactivation process. |
| Potential Therapeutics | The techniques developed by Jeannie Lee’s lab may lead to therapies for genetic disorders stemming from mutations on the X chromosome, including Fragile X and Rett syndromes. |
| Clinical Implications | Understanding how to unsilence inactivated X chromosomes could provide cures with minimal side effects for conditions that affect genes on the X chromosome. |
Summary
X-chromosome inactivation is a critical biological process that allows female cells to balance gene expression from two X chromosomes. This complex mechanism, studied extensively by Jeannie T. Lee’s lab, reveals how a gelatinous substance separates chromosomal structures and facilitates the inactivation of one X chromosome. The pivotal role of the Xist gene marks a significant breakthrough in understanding this process, which not only answers long-standing questions in cell biology but also opens doors for potential therapies for genetic disorders like Fragile X and Rett syndromes. The innovative findings could ultimately lead to effective treatments, showcasing the importance of ongoing research in genetics.